
Blog
Manganese (Mn) in Water and Its Removal: A Growing Concern
Authors – Colman Lynch (Process Scientist) and Danny Flynn (Principal Process Design Engineer).
In this post, we will discuss Manganese (Mn) in water and its removal. Manganese (Mn) is a naturally occurring metal found in surface and groundwater sources, but at high levels of exposure, it poses significant toxicity risks. Human activities, such as conifer planting, liming, artificial fertiliser use, drainage ditch construction, and ploughing, contribute to heightened Mn concentrations in water run-off. Additionally, Mn can enter water systems through the use of the fuel additive methylcyclopentadienyl manganese tricarbonyl (MMT), which releases Mn particles into the soil, eventually making their way into waterbodies (Ljung & Vahter, 2007; O’Neal & Zheng, 2015).
Regulatory Standards and Health Impacts
In Ireland, the Prescribed Concentration Value (PCV) for Mn in drinking water is set at 0.05mg/L under S.I. No. 122 of the 2014 European Union (Drinking Water) Regulations. The Agency for Toxic Substances and Disease Registry (ATSDR) recommends that daily manganese intake should not exceed 5mg. Despite these regulations, elevated Mn levels pose health risks, particularly to vulnerable populations like children. Studies have shown that Mn exposure in drinking water is linked to lower IQ scores and hyperactive behaviour in children (Tobiason et al., 2016), with increased risks of attention deficit hyperactivity disorder (ADHD) observed in Denmark (Schullehner et al., 2020).
Manganese and “Do Not Consume” Events in Ireland
In recent years, Ireland has experienced a rise in “do not consume” (DNC) events, driven by spikes in Mn levels in surface water sources. Sites found to have elevated Mn levels greater than 50 µg/l in treated water are to be reported by Uisce Eireann (UE) to the HSE, as this is above the legal limit. HSE guidance is that water with Mn greater than 120 µg/l is unsafe for human consumption, however Mn levels of 50 µg/l would not be palatable due to taste and colour.
Challenges in Water Treatment for Manganese (Mn) in Water and its Removal
Mn contamination is not only a health issue but also a challenge for water treatment. Mn exists in different forms in surface water—particulate, colloidal, and dissolved. While particulate and colloidal Manganese (Mn) can be removed through coagulation and filtration, dissolved Mn requires oxidation (Wu et al., 2023). This process converts dissolved Mn(II) into manganese dioxide (MnO2), which can then be filtered out. Conventional water treatment methods, such as rapid gravity filtration, have proven ineffective at removing dissolved Mn, necessitating innovative solutions. Insoluble Mn can be easily removed using coagulation, filtration, and sedimentation processes, but these are not effective for removing the soluble forms of Mn (Raveendran et al., 2001). Mn is typically removed by oxidising the dissolved Mn(II) form into MnO2 precipitates, followed by coagulation, flocculation, clarification, and filtration (Zogo et al., 2011). Elevated Mn levels in surface water differ from those in groundwater sources and require different treatment approaches.
Forms of Manganese and Treatment Approaches
Manganese in water and its removal presents various challenges depending on its form. Mn can be insoluble or soluble, and inorganic or organically bound. Insoluble Mn can be removed by coagulation, clarification, and filtration processes. Inorganic soluble Mn can be easily oxidised by chemical or manganese dioxide filter media. However, organically bound soluble Mn is more difficult to oxidise and requires specialised process design. The oxidation of Mn is complicated by its varying oxidation states, which depend on environmental pH and redox potential. In well-aerated surface waters, Mn typically exists in its +2 (dissolved) or +4 (particulate) states, but under anaerobic conditions, Mn(II) levels increase in deeper, oxygen-depleted waters (Linnik, 2018). These conditions often occur in eutrophic lakes, where stratification prevents mixing between water layers. When reservoirs experience drawdown, such as during droughts, Mn-rich water from the hypolimnion may be brought to the surface, causing sudden spikes in Mn levels. This occurred in the UK during 2021, where pronounced Mn spikes were attributed to reservoir drawdown. Such events present serious challenges for water treatment plants, as rapid changes in water quality can overwhelm standard treatment processes (Semasinghe & Rousso, 2023).
The Impact of Climate Change on Manganese (Mn) in Water and its Removal
Climate change exacerbates these challenges, as rising temperatures and increased solar radiation impact surface water chemistry. Stratification is a common natural phenomenon in reservoirs and deep lakes. This creates a density and temperature structure with a well-mixed surface layer at the top separated from a deeper hypolimnion layer by a layer of strong temperature gradient (Sahoo & Luketina, 2003). Lake turnover occurs seasonally, usually during the Spring and in the Autumn. Lake turnovers occur rapidly and are usually a result of sudden temperature changes or water level rise because of heavy rainfall. Drought followed by periods of heavy rainfall may also cause lake turnover as algal blooms fed by nutrient run-off die off and deplete oxygen from the lake and oxidise sediments such as Mn. Warmer winters in the Northern Hemisphere have been preventing complete lake turnover (holomixis), which is essential for replenishing oxygen levels in deep water layers (Yankova et al., 2017). This lack of turnover can lead to anoxic conditions in the hypolimnion, which can release Mn and other metals from sediments into the water, further degrading water quality (Visser et al., 2015).
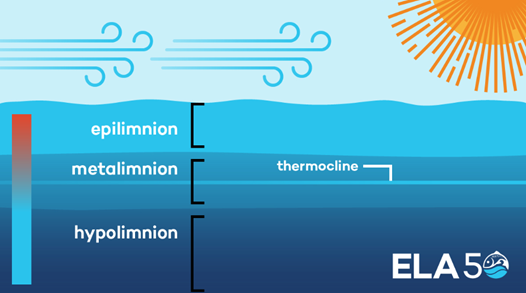
Figure 1: Layers of water stratification in a lake
Water Stratification
Solar radiation warms the surface waters in the epilimnion during the summer, and wind and waves mix the epilimnion waters with the metalimnion. Stratification stabilises with solar radiation, with the more dense, colder waters staying on the bottom in the hypolimnion layer. The greater the temperature difference between the epilimnion and the hypolimnion the more stable the stratification becomes, with the mixing ability of a lake or reservoir determined by the stability of thermal stratification (Fafard, 2018). The epilimnion will cool until the entire lake is the same temperature also known as isothermal. This isothermal stage will allow the lake to turnover. Turnover of a lake occurs when the entire volume of water in a lake is mixed by wind. This may only occur when the entire lake is the same temperature. In a reservoir in Malaysia facing problems with high iron and Mn concentrations it was found that the level of stratification varied at different times of the year. During the most intense period of stratification, dissolved oxygen levels were found to significantly decrease with depth, while iron and Mn concentrations were at very high levels. It was found that high rainfall events temporarily led to the natural destratification of the reservoir, however this was only a short reprieve before concentrations of iron and Mn again increased due to thermal stratification (Baharim et al., 2011). Wind turbulence, and inflows to the reservoir may also provide natural means to prevent the stabilisation of the water column in the reservoir.
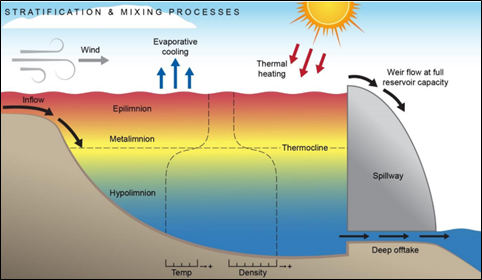
Figure 2: Stratification and mixing processes in reservoir
Innovative Solutions for Manganese (Mn) in Water and its Removal
Coftec recognises the weaknesses of certain Mn treatment processes, whether they are THM formation for Chlorine Oxidisation, purple colour risk from Potassium Permanganate Oxidisation, or pH control and exhaustion of media when using Manganese Dioxide filter media. By introducing the principle of Ozone & Biological GAC, certainty can be increased of the effectiveness of manganese oxidation. Inorganic soluble Mn is easily oxidised by Ozone. Ozone will also break down long-chained organically bound manganese molecules to short-chained molecules, which are used as a food source for microbiological growth in the biological GAC. By installing Ozone upfront of GAC, higher levels of dissolved oxygen (DO) are experienced in the water, thus aiding biological growth. Ozone decomposes in water, producing hydroxyl radicals, which are the most potent oxidising agents in water treatment (Rodríguez et al., 2008). The oxidation of dissolved inorganic Mn is more effective with ozone when applied to treated water rather than raw water (McKnight et al., 1993). Following ozonation, adsorption onto a treated GAC system occurs, with research indicating that ozonation before GAC treatment has no adverse impact on GAC quality, even after ten reactivation cycles (Boere, 1992).
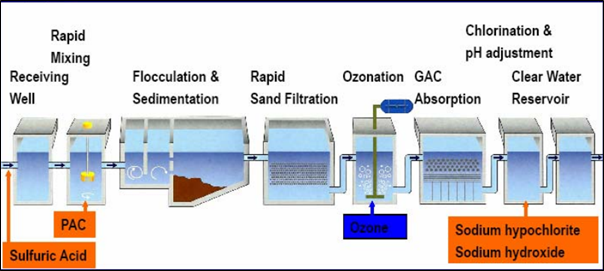
Figure 3: Ozonation and GAC water treatment process
The Risks and Costs of Manganese (Mn) in Water and its Removal
Effective Mn removal strategies include oxidation using chlorine, potassium permanganate, ozone, and biological granular activated carbon (GAC). However, these methods must be used carefully, as prechlorination can lead to the formation of disinfection byproducts (DBPs), such as trihalomethanes (THMs), which are harmful to human health (LaBerge, 2014). Mn greensand filtration beds offer an effective method for removing Mn, though they require activation with potassium permanganate (Outram et al., 2018). Potassium permanganate (KMnO4) is a very effective oxidising agent of Mn in surface water which is commonly used in combination with greensand media however pH is a limiting factor, with pH treatment required, which is an additional operational cost. KMnO4 is an oxidant, poisonous, and an irritant to skin, with care required for its handling as it comes in a powdered or liquid form for dosing. KMnO4 imparts a pink colour to water and stains skin. A balance is required at dosing to prevent the water from going a dark purple colour which may cause consumer complaints. Furthermore, issues with fluctuating Mn levels in water make it impossible to set the optimum dosage level for KMnO4 in many water treatment facilities (Burns, 1998). KMnO4 is also an expensive product due to its long production process which requires significant energy (Zhao et al., 2011).

Figure 4: Potassium Permanganate (KMnO4) crystals
Customer Complaints and Distribution System Challenges
Increased Mn levels can lead to customer complaints about water discoloration, metallic taste, and scaling of pipes and appliances. Mn may also stain laundry where bleach is used and can cause the growth of bacterial black slimes in water supply pipes, reducing the chlorine residual. Mn concentrations as low as 0.03mg/L can prompt complaints, particularly when black or brown water is observed (Raveendran et al., 2001). The US Environmental Protection Agency (USEPA) has set a secondary maximum contaminant level (SMCL) of 0.05mg/L for Mn, with a health advisory level of 0.3mg/L (Tobiason et al., 2016). Accumulation of Mn has also been linked to lead (Pb) release (Earle et al., 2023). Deposits of residual Mn(II) from treated water may also deposit in drinking water distribution systems and lead to the formation of disinfection by-products (DBPs) in chlorinated drinking water distribution systems, such as trihalomethanes (THMs). In a 100 μg/L Mn system, the presence of THMs was 20% higher than an Mn-free system after 150 days of operation. THMs are carcinogenic disinfection byproducts (Yu et al., 2021).
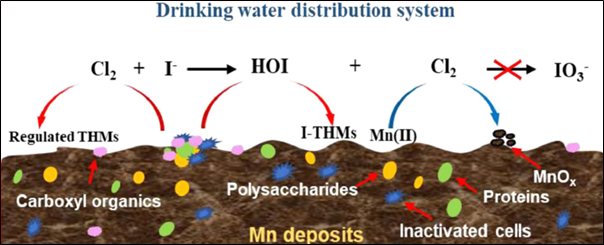
Figure 5: Formation of THM’s from Mn deposits in a drinking water distribution system (Yu et al., 2021).
Coftec’s Expertise
At Coftec, we understand the complexities and difficulties faced when dealing with manganese and other recalcitrant substances in raw and wastewater sources. We have experience in addressing a variety of contaminants in drinking water, including manganese. Our multi-disciplinary team of scientists and engineers offer a proven track record of leveraging their expertise and experience in finding innovative and practical solutions to the problems such as excess levels of manganese faced at water treatment plants.
Contact Coftec
Contact Coftec whether designing a new facility or refurbishing, expanding or bringing an existing facility up to regulatory standards to discuss how we can help in your project.
References
Baharim, N.H., Ismail, R. and Omar, M.H. (2011) “Effects of Thermal Stratification on the Concentration of Iron and Manganese in a Tropical Water Supply Reservoir,” Sains Malaysiana, (40), pp. 821–825.
Boere, J.A. (1992) “Combined use of ozone and granular activated carbon (GAC) in potable water treatment; effects on GAC quality after reactivation,” Ozone: Science & Engineering, 14(2), pp. 123–137. Available at: https://doi.org/10.1080/01919519208552294.
Burns, F.L. (1998) “Case study: Automatic reservoir aeration to control manganese in Raw Water Maryborough Town Water Supply Queensland, Australia,” Water Science and Technology, 37(2). Available at: https://doi.org/10.1016/s0273-1223(98)00037-7.
Earle, M.R., Stoddart, A.K. and Gagnon, G.A. (2023) ‘Raw water biofiltration for surface water manganese control’, Scientific Reports, 13(1). doi:10.1038/s41598-023-36348-1.
Fafard, P. (2018) “How and Why Lakes Stratify and Turn Over: We explain the science behind the phenomena,” International Institute for Sustainable Development. International Institute for Sustainable Development, 16 May. Available at: https://www.iisd.org/ela/blog/commentary/lakes-stratify-turn-explain-science-behind-phenomena
LaBerge, E.R. (2014) Study of the formation and control of disinfection by-products originating from a surface water supply on the volcanic island of Guam. thesis. University of Central Florida.
Linnik, P.N. (2018) ‘Coexisting manganese species in surface water of the Ukraine and their significance for aquatic ecosystems’, Russian Journal of General Chemistry, 88(13), pp. 2918–2927. doi:10.1134/s1070363218130157.
Ljung, K. and Vahter, M. (2007) ‘Time to re-evaluate the guideline value for manganese in drinking water?’, Environmental Health Perspectives, 115(11), pp. 1533–1538. doi:10.1289/ehp.10316.
McKnight, K.F. et al. (1993) “Comparison of ozone efficiency for manganese oxidation between raw and settled water,” Ozone: Science & Engineering, 15(4), pp. 331–341. Available at: https://doi.org/10.1080/01919519308552493.
O’Neal, S.L. and Zheng, W. (2015) ‘Manganese toxicity upon overexposure: A decade in Review’, Current Environmental Health Reports, 2(3), pp. 315–328. doi:10.1007/s40572-015-0056-x.
Outram, J.G., Couperthwaite, S.J. and Millar, G.J. (2018) “Investigation of manganese Greensand activation by various oxidants,” Journal of Environmental Chemical Engineering, 6(4), pp. 4130–4143. Available at: https://doi.org/10.1016/j.jece.2018.05.060.
Raveendran, R., Ashworth, B. and Chatelier, B. (2001) ‘64th Annual Water Industry Engineers and Operators’ Conference All Seasons International Hotel – Bendigo 5 and 6 September, 2001’, in MANGANESE REMOVAL IN DRINKING WATER SYSTEMS. Bendigo, Bendigo: South Gippsland Water, pp. 92–100. Available at: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=1fbfe6f6205ca58a885ccfdfb3bdde61aa6d1f38
Rodríguez, A. et al. (2008) “Ozone-based technologies in water and wastewater treatment,” The Handbook of Environmental Chemistry, pp. 127–175. Available at: https://doi.org/10.1007/978-3-540-79210-9_4.
Sahoo, G.B. and Luketina, D. (2003) ‘Modeling of bubble plume design and oxygen transfer for Reservoir Restoration’, Water Research, 37(2), pp. 393–401. doi:10.1016/s0043-1354(02)00283-x.
Schullehner, J. et al. (2020) ‘Exposure to manganese in drinking water during childhood and association with attention-deficit hyperactivity disorder: A nationwide cohort study’, Environmental Health Perspectives, 128(9). doi:10.1289/ehp6391.
Semasinghe, C. and Rousso, B.Z. (2023) ‘In-lake mechanisms for Manganese Control—a systematic literature review’, Sustainability, 15(11), p. 8785. doi:10.3390/su15118785.
Tobiason, J.E. et al. (2016) ‘Manganese removal from drinking water sources’, Current Pollution Reports, 2(3), pp. 168–177. doi:10.1007/s40726-016-0036-2.
Visser, P.M. et al. (2015) ‘Artificial mixing to control cyanobacterial blooms: A Review’, Aquatic Ecology, 50(3), pp. 423–441. doi:10.1007/s10452-015-9537-0.
Wu, Y.-C. et al. (2023) ‘Water treatment of manganese oxides and organic matter through pre-oxidation and coagulation/sedimentation’, 2023 IEEE 5th Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability [Preprint]. doi:10.3390/engproc2023055059.
Yankova, Y. et al. (2017) ‘Abrupt stop of deep water turnover with Lake warming: Drastic consequences for algal primary producers’, Scientific Reports, 7(1). doi:10.1038/s41598-017-13159-9.
Yu, Y. et al. (2021) “Trihalomethanes Formation enhanced by manganese chlorination and deposition in plastic drinking water pipes,” Water Research, 204, p. 117582. Available at: https://doi.org/10.1016/j.watres.2021.117582.
Zhao, F.-W., Li, X. and Graham, N. (2011) “The application of potassium manganate in water treatment,” Water Supply, 11(5), pp. 612–620. Available at: https://doi.org/10.2166/ws.2011.023.
Zogo, D. et al. (2011) ‘Effect of pre-chlorination on the efficiency of iron and manganese removal from surface water by coagulation-flocculation using aluminium sulphate: Case of the Okpara Dam in the Republic of Benin’, International Journal of Biological and Chemical Sciences, 4(6). doi:10.4314/ijbcs.v4i6.64983.
